Trusted Clinical Trial Partner for over 15 years
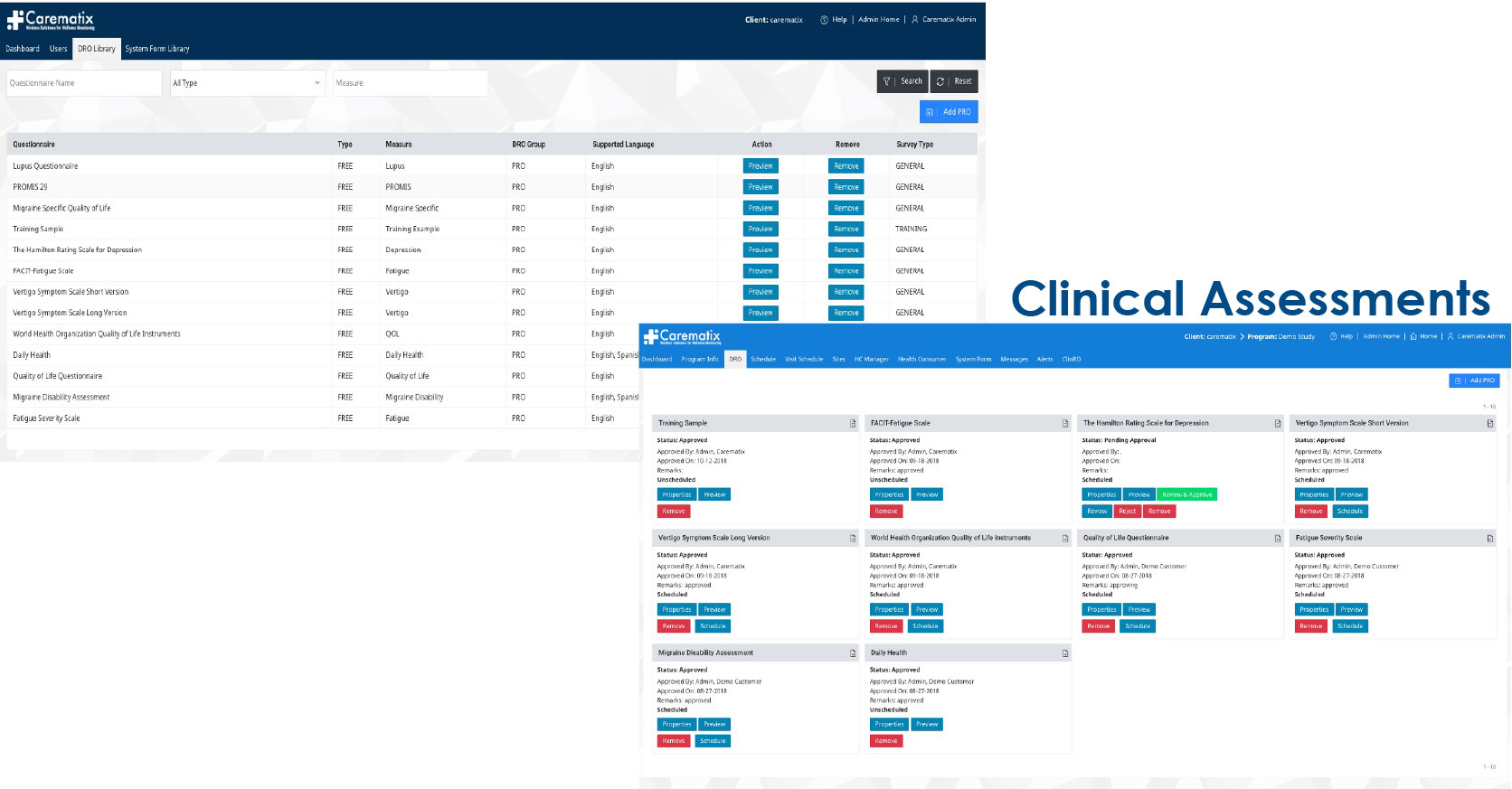
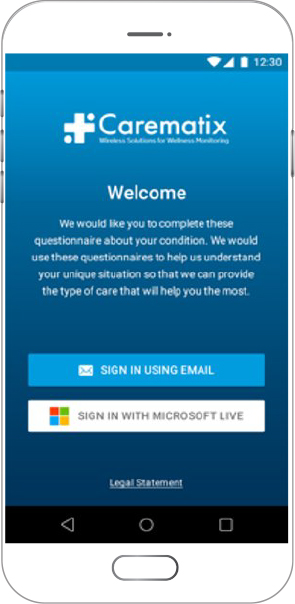
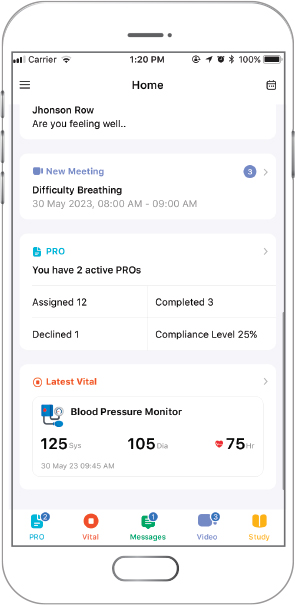
A Patient-centric Clinical Trial (DRO) Platform
developed with Pharma oversight designed to:
- Improve efficiency
- Increase diversity
- Work across geographies and on all platforms.
- Available for iOS, Android and Web
The solutions comply with FDA, CE, GCP, and HIPAA regulations.
Carematix has 5 FDA 510(k) Clearances.